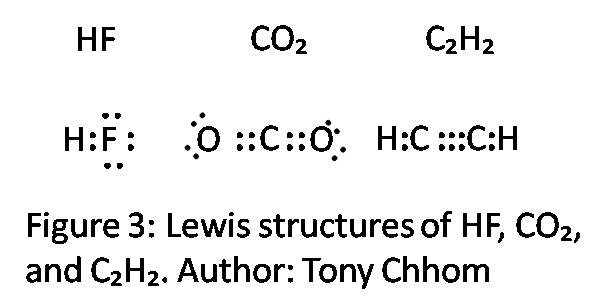
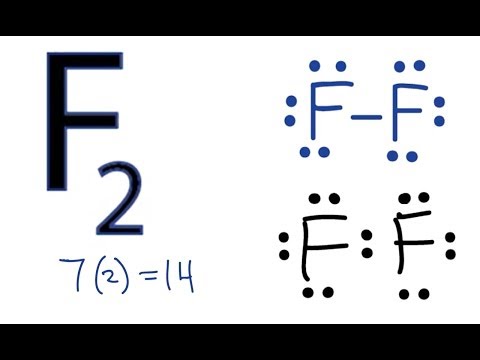
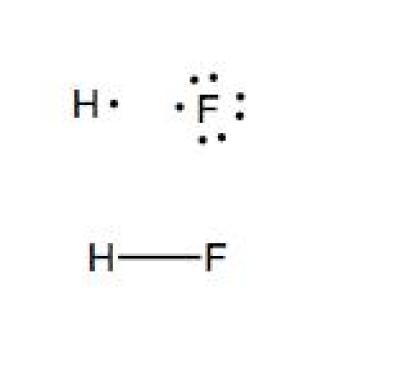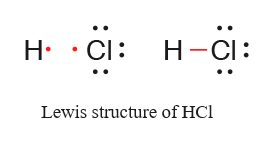Hf Electron Dot Structure

Hf is very similar to hf and hcl.
Hf electron dot structure. The structure on the right is the lewis electron structure or lewis structure for h 2 o. The warped side of the universe. Kip thorne at cardiff university duration. Lewis who described them in a 1916 article titled the atom and the molecule lewis structures depict the bonds between atoms of a molecule as well as any unbonded electron pairs.
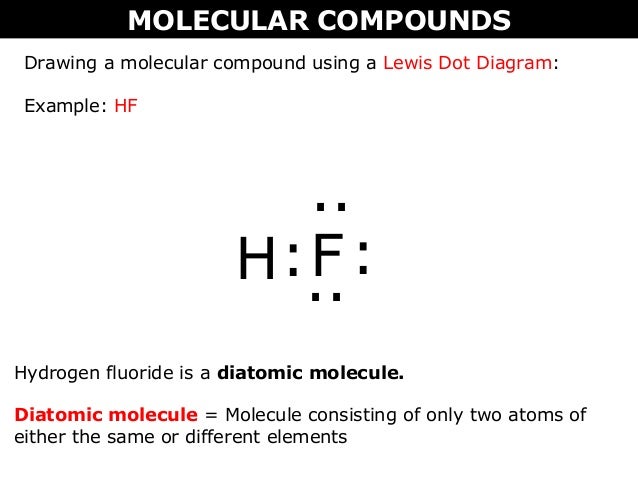
Wayne breslyn 38 890 views. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Drawing the lewis structure for hf. The lewis dot structure the lewis structure will help us know the location of electrons around the atoms in the molecule and how the atoms are organized.
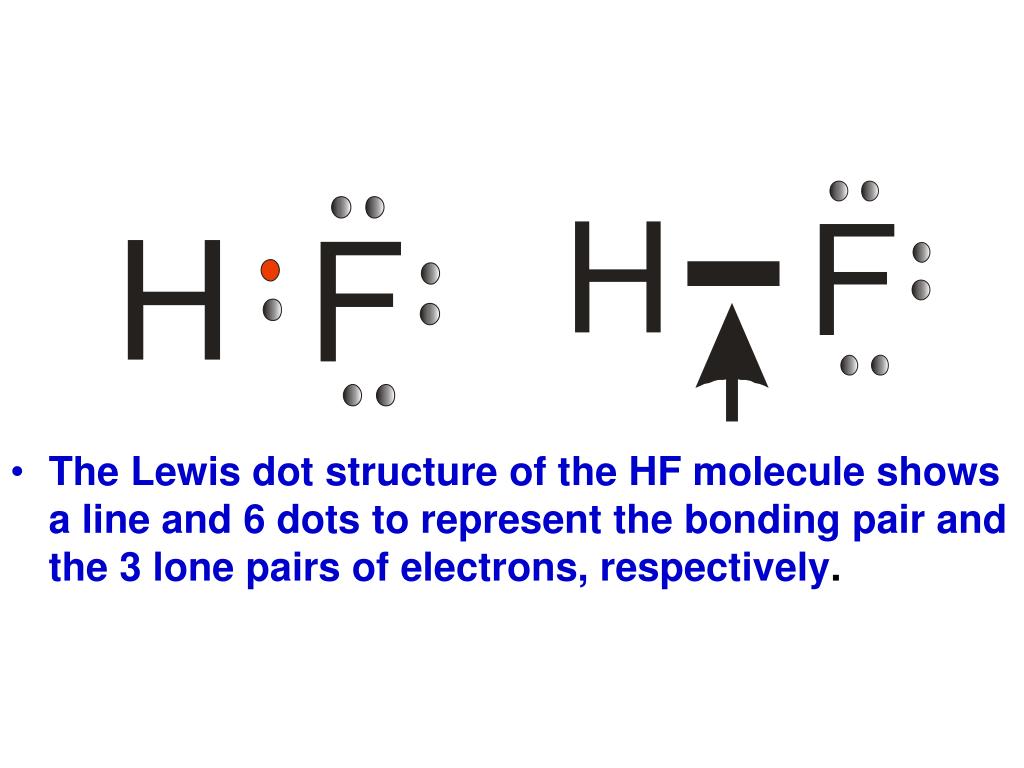
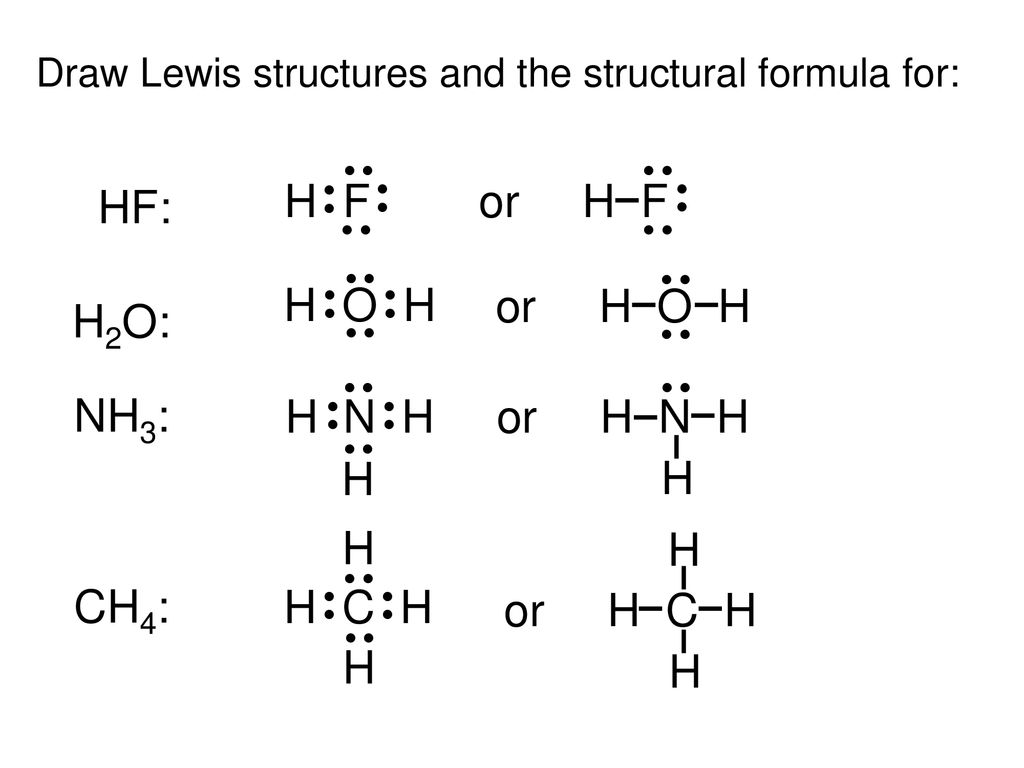
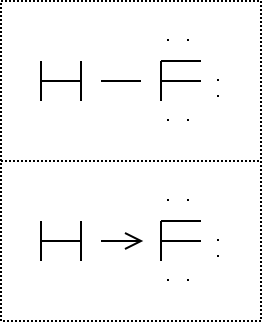
A step by step explanation of how to write the hf lewis structure hydrofluoric acid. The electron dot symbol or electron dot structure of an element shows the valence electrons as dots. With the lewis structure for hf remember that hydrogen only needs 2 valence electrons to have a full outer shell. Hydrogen has 1 valence electron and fluorine in group 7 with f and cl has 7 valence electrons.
Hf lewis structure how to draw the dot structure for hf duration. A lewis structure is a graphic representation of the electron distribution around atoms. With two bonding pairs and two lone pairs the oxygen atom has now completed its octet. First we count the valence electrons for hf using the periodic table.
Hydrogen atoms can also combine with fluorine atoms to form hf molecules. Moreover by sharing a bonding pair with oxygen each hydrogen atom now has a full valence shell of two electrons. Atomic structure of hafnium. Electrons that are paired in an orbital are shown as a pair of dots.
From the structure it is clear that fluorine being one of the most electronegative elements in the periodic table will try to pull the shared pair of electrons towards itself and not allow equal sharing between h and f. Lewis structures also known as electron dot structures are named after gilbert n. Cross section thermal neutron capture σ a barns. H f h f or h f carbon is in group 4a on the periodic table so we predict that it has four valence.
Electrons per energy level.